Lithium iron phosphate (LiFePO₄, LFP) is a well-established cathode material in lithium-ion battery research due to its excellent electrochemical stability, intrinsic safety, non-toxicity, and low cost. Beyond its commercial success, LiFePO₄ has attracted increasing attention as a reference material due to its highly stable and reproducible electrode potential in non-aqueous lithium-based electrochemical systems.
In conventional electrochemical measurements, metallic lithium is most commonly used as the reference electrode. However, metallic lithium suffers from significant drawbacks, including high chemical reactivity toward certain electrolytes and additives such as acetonitrile, a very low electrochemical potential that can unintentionally reduce many substances, and the occurrence of spikes in potential profiles that are readily observable in the differential capacity (Figure 1). These effects can lead to limitations in material selection and unintended measurement deviations.
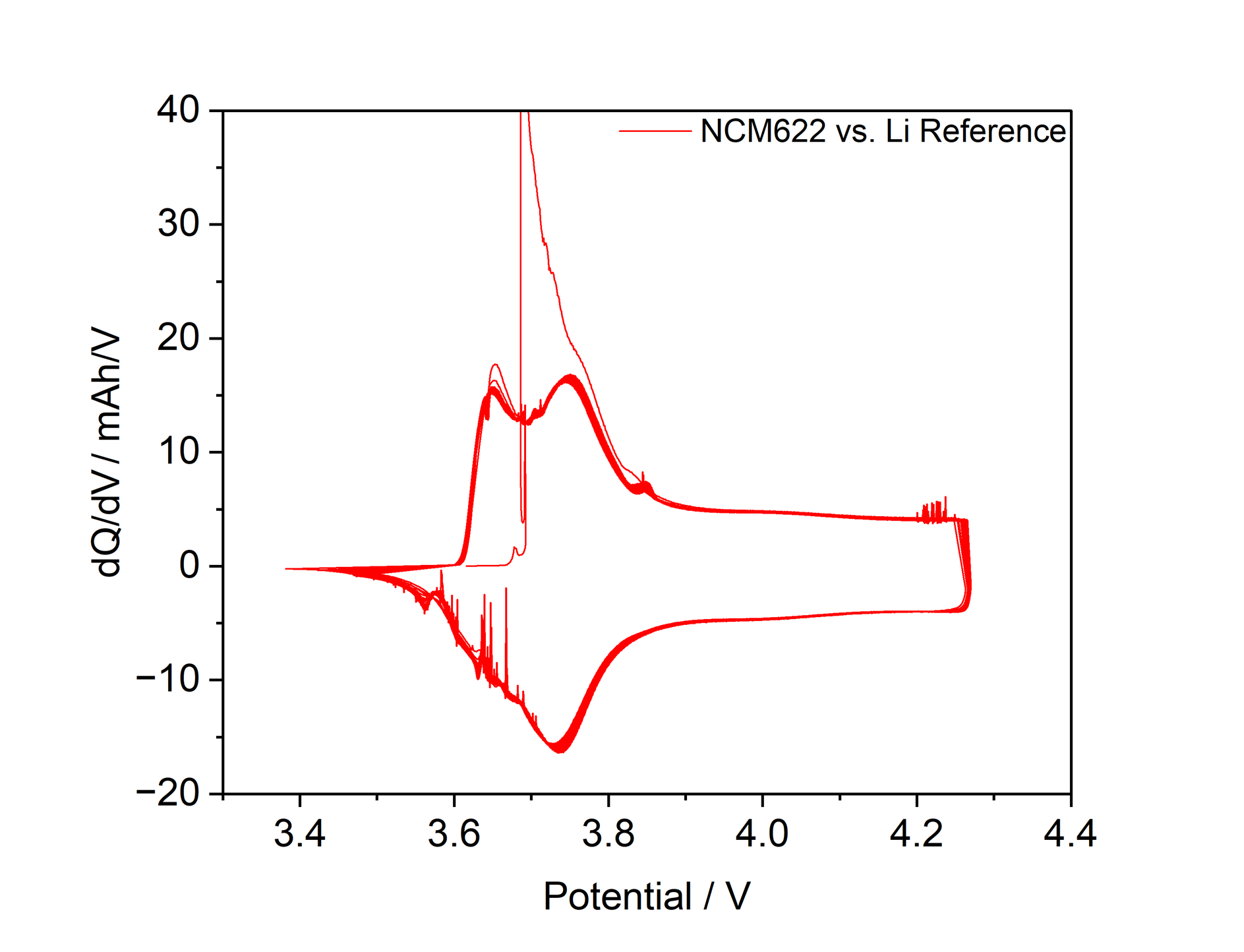
Figure 1: Differential Capacity of NCM622 in a NCM622 vs. Graphite cell with Li-Reference cycling at C/10 for 1000 h. The spikes are attributed to unwanted side reactions associated with the use of metallic lithium as the reference electrode.
Approach
This application note presents partially delithiated LiFePO₄ as a chemically stable and reliable alternative to metallic lithium. Controlled chemical delithiation of LFP enables the establishment of a well-defined, constant electrode potential of approximately +3.420 V vs. Li, which lies within the operating voltage window of most lithium battery materials (Figure 2). This approach eliminates the need for electrochemical delithiation of LFP as a reference in a three-electrode setup, removing a processing step that would otherwise affect the balance between the working and counter electrodes. As a result, the reference electrode is immediately ready for use and provides long-term potential stability, free of parasitic side reactions or measurement inaccuracies.
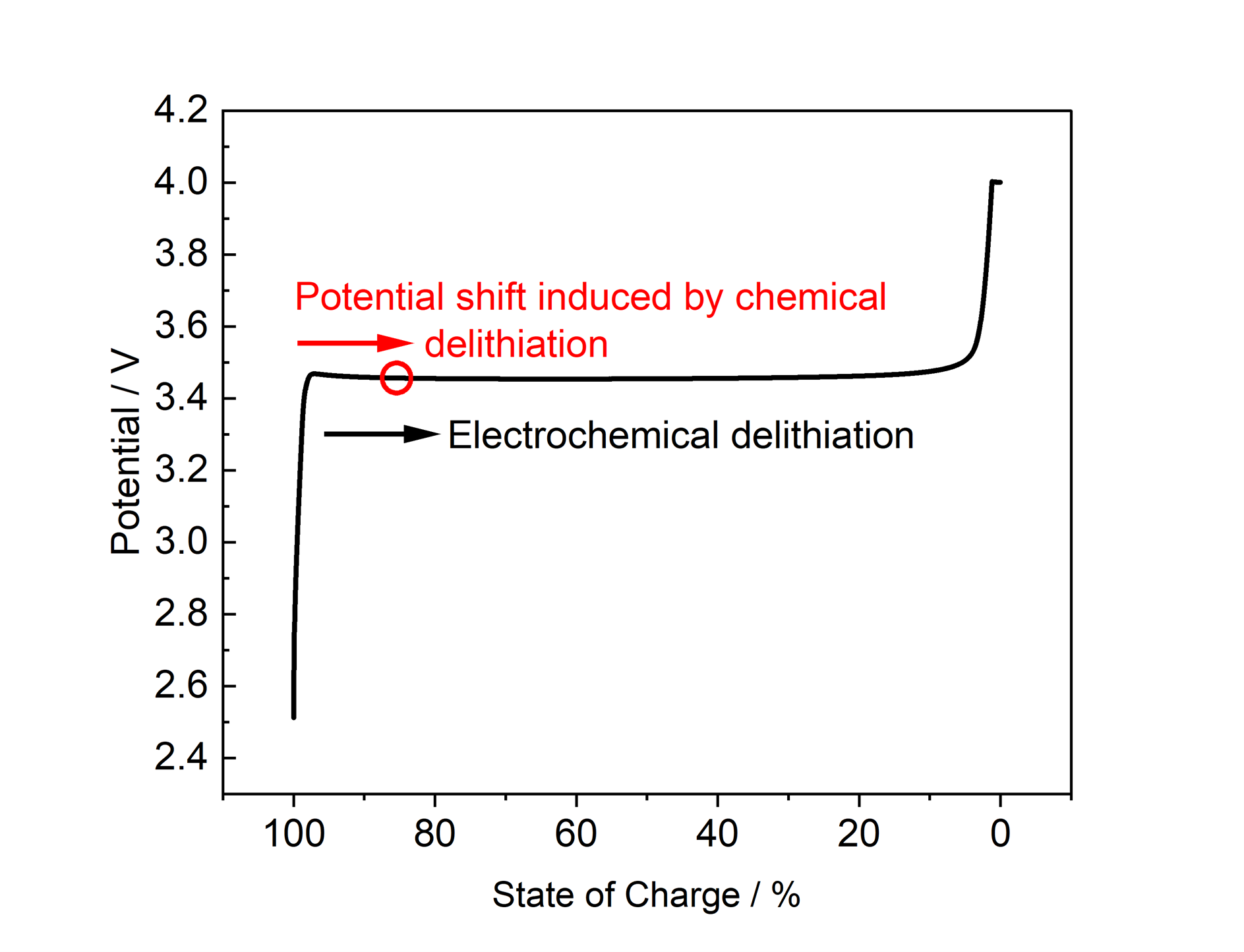
Figure 2: Potential of LFP as a function of state of charge during electrochemical cycling of LFP vs. lithium at C/10. Chemical delithiation of LFP results in a shift of the potential within a stable window.
Results:
The electrochemical performance and long-term stability of partially delithiated LiFePO₄ under practical operating conditions are investigated. The results depicted in Figure 3 demonstrate that LFP-based reference electrodes exhibit excellent chemical inertness toward common organic electrolytes, superior long-term potential stability, and enhanced safety compared to lithium metal references. Additionally, the voltage spikes observed in measurements with a metallic Li-reference (Figure 1) do not occur.
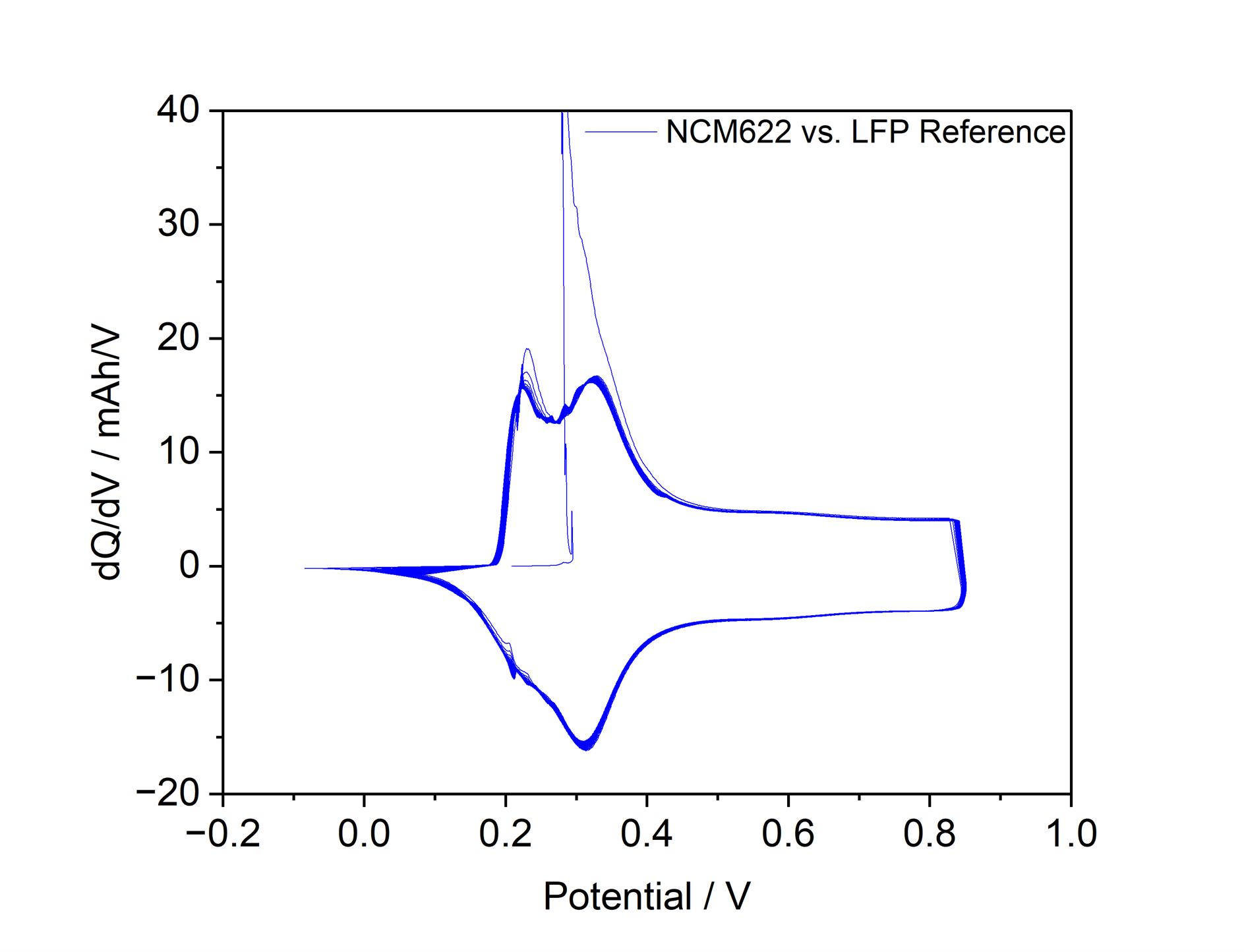
Figure 3: Differential Capacity of NCM622 in a NCM622 vs. Graphite Cell with LFP-Reference cycling at C/10 for 1000 h. No voltage spikes can be observed.
In direct comparison with metallic lithium, LiFePO₄ exhibits excellent reproducibility as a reference electrode (Figure 4). This is particularly evident in differential capacity (dQ/dV) measurements of graphite electrodes versus LiFePO₄, which show well-defined and highly reproducible features. Unlike lithium metal references, LiFePO₄ does not suffer from measurement artifacts and effectively eliminates voltage spikes. As a result, LiFePO₄ enables precise and reliable electrochemical analysis, particularly for long-term measurements.
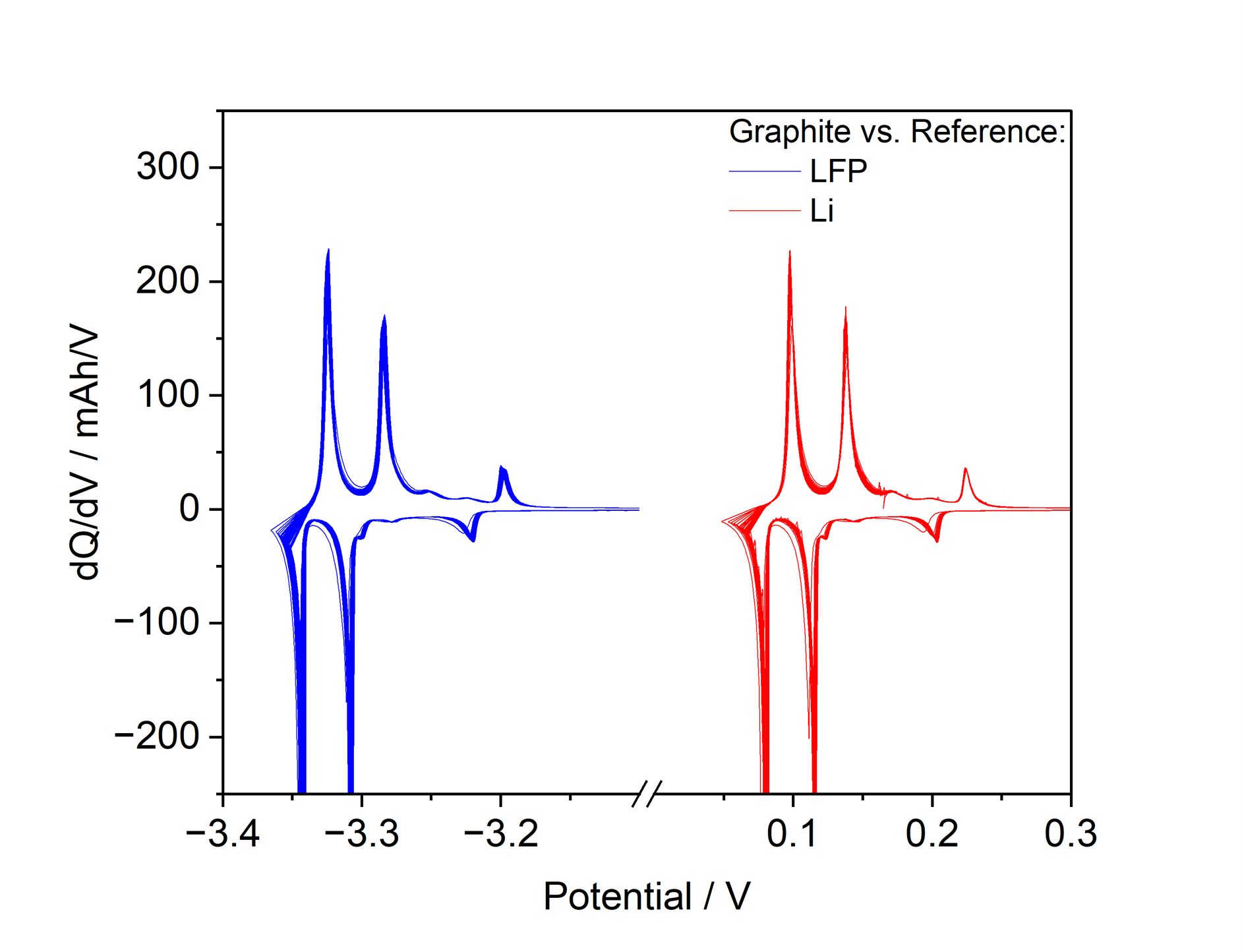
Figure 4: Differential Capacity of Graphite in a NCM622 vs. Graphite Cell with LFP- and Li-Reference cycling at C/10 for 1000h.
Conclusion:
The use of LiFePO₄ as a reference electrode significantly enhances the safety, precision, and reproducibility of electrochemical measurements. It enables investigation of a wide range of electrode materials, electrolytes, and additives while maintaining exceptionally high reproducibility and long-term stability. This makes LiFePO₄ a reliable reference electrode for studying lithium-ion-batteries.
The advantages of using chemically delithiated LFP as a reference material are, among others:
- Chemical stability against most battery components, and thus fewer side reactions
- Ready-to-use electrode; no in-situ delithiation required
- Excellent long-term stability, demonstrated over 1000+ hours.
_
by Jan Römer et al.
Related products:
 Reference ring, LFP, modified (10 pcs), ECC1-00-0482-C/X
Reference ring, LFP, modified (10 pcs), ECC1-00-0482-C/X
 Insulation sleeve PP (LFP-Reference, Separator GF/A) (10 pcs), ECC1-00-0450-Q/X
Insulation sleeve PP (LFP-Reference, Separator GF/A) (10 pcs), ECC1-00-0450-Q/X
 Insulation sleeve PP (LFP-Reference, Separator FS-5P) (10 pcs), ECC1-00-0450-R/X
Insulation sleeve PP (LFP-Reference, Separator FS-5P) (10 pcs), ECC1-00-0450-R/X
 Insulation sleeve PP (LFP-Reference, Separator QT17) (10 pcs), ECC1-00-0450-S/X
Insulation sleeve PP (LFP-Reference, Separator QT17) (10 pcs), ECC1-00-0450-S/X




Comments are closed.